Introduction
Materials and Methods
Plant Materials
Morphological Analysis
Stomatal Density and Length
Observation of the Tissue Structure of the Leaf Cross Section
Scanning Electron Microscopy
Flow Cytometric Analysis
High-Performance Liquid Chromatography Carotenoid Analysis
DNA Extraction and Simple Sequence Repeat Analysis
Statistical Analysis
Results
Morphological Characteristics of the Fruit
Leaf Morphology
Morphology of Mature Pollen using Scanning Electron Microscopy
Ploidy Determination
Identification of Carotenoids
Characterization of the Nuclear, Chloroplast, and Mitochondrial Genomes
Discussion
Introduction
Citrus is extensively planted in tropical and subtropical regions worldwide and includes five main economic groups: orange (Citrus sinensis), mandarin (C. reticulata), grapefruit (C. paradisi), pummelo (C. maxima), and lemon (C. limon). Over the past 30 years, citrus breeding improvement has been concentrated on seedlessness, ease of peeling, strong flavor and aroma aspects. Bud sport selection and cross-breeding are the two conventional breeding methods and have been used to develop more than 100 citrus cultivars (Deng, 2005). Citrus chimera breeding is an emerging breeding strategy that has made great progress in fresh markets.
Generally, the shoot apical meristem (SAM) of plants is composed of cells with two or more different genotypes. In dicotyledon plants, the SAM consists of three cell layers: dermatogen initial (the outermost layer, L1), periblem initial (middle layer, L2), and plerome initial (the innermost layer, L3) (Schmidt, 1924). In citrus fruit, the juice sac and epidermal pericarp develop from L1; the seed, segment wall, hypoderm, and mesocarp of the pericarp are produced by L2; and the L3 cell layer is often involved in vascular bundle formation (Ohtsu and Kuhara, 1994). Artificial plant chimeras develop spontaneously as intervariety and interspecies chimeras invitro, although the use of layer-specific mutations to induce phenotypic trait changes is another method to develop a periclinal chimera. Based on previous studies, the research in this field can be divided into two major branches; the first branch seeks to breed new varieties, and the second branch investigates the different genotype cell layer interactions using natural and synthetic plant chimeras. At present, a grafted chimera is an ideal model to investigate stock-scion interactions and different genotype cell layer displacements. Cao et al. (2016) studied the inner mechanism between stock and scion in the artificial plant chimeras Brassica juncea and Brassica oleracea. They reported that short-distance mobile communication via small RNAs could regulate the expression of related genes and lead to different phenotypes. Additionally, DNA methylation and changes in DNA methylation patterns in transposons play a role in graft-induced phenotypic variations. Generation of grafting mutations may be the cause of phenotypic variation through changes in DNA methylation patterns in transposons (Li et al., 2013).
To date, few reports have investigated xylophyta chimeras. Citrus is one of the most commonly described xylophyta types. The occurrence of citrus chimeras usually emerges from an adventitious bud, which is derived from the callus tissue in the graft junction of the stock and scion, and typically the callus is caused by unintentional injury of the scion. As early as 1674, ‘Bizzaria’ was reported as the first chimera that arose from a graft union of C. medica and C. sinensis (Tilney-Bassett, 1986). Later, some citrus-grafted chimeras were successively released (Sugawara et al., 2002; Zhang et al., 2007, 2015). More studies have investigated artificial synthetic citrus grafting chimeras in Japan, and some new germplasms with high quality and disease resistance have been developed. For example, Kuhara (1989) successfully synthesized the first citrus chimera (C. sinensis + C. natsudaidai), and Ohtsu and Kuhara (1994) also put forward two artificial citrus chimeras named ‘NFF’ and ‘FNN’ from the synthetic donor plants ‘Fukuhara’ sweet orange and ‘Kawano’ natsudaidai.
C. changshan-huyou (abbreviated CH), which is a precious citrus germplasm resource native to Changshan county of Zhejiang province in China, is widely cultivated for its large fruit, high yield, excellent quality, and unique flavor. In the 1990s, CH was used as a scion and was top grafted upon the satsuma mandarin stock. Then, a natural chimera named ‘Hongrou Huyou’ (HH) adventitiously arose at the grafted junction of CH and the satsuma mandarin (C. unshiu), and showed deep-orange color and juicy flesh, strong flavor, and good storage ability.
In this study, to explore the genetic composition of HH, we performed a characteristic evaluation of the morphology, cytology, and molecular markers of this chimera.
Materials and Methods
Plant Materials
The chimera HH arose at a graft union zone where a bud of CH was grafted onto a satsuma mandarin in the 1990s. Recently, HH was discovered in an orchard in Changshan county of Zhejiang province during a bud mutation investigation. The fruit on the branch with the pericarp was similar to that of CH, and the flesh was similar to that of the satsuma mandarin. The trees of the three varieties were planted in the same orchard with conventional management. All samples, including the fruits and leaves, were picked from the regrafted trees (with Poncirus trifoliata as the rootstock)for decades. The picked fruits of HH were immediately divided into four parts: outer pericarp (epidermis and flavedo), albedo, segment wall, and juice sac. Then, the fruit parts were quickly frozen in liquid nitrogen and stored at ‑ 80°C. Because CH was extensively used as a high scion to replace the ‘Owari’ satsuma mandarin (OW) in the 1990s, we conjectured that HH was a peripheral chimera formed by grafting; therefore, CH and OW are assumed to be the donor plants of HH.
Morphological Analysis
Both length and width of leaves and fruits of the HH and donor plants were measured. Current spring shoots from the outer layer of the middle part of the canopy were collected in summer, and mature fruits of the same size were selected. Leaf and fruit samples were taken from three trees, 10 samples each.
Stomatal Density and Length
The leaves were collected from mature spring shoots, and a thin layer was evenly smeared with nail polish along the two sides of the main vein on the back of the leaf (approximately 5 ‑ 10 mm). After 5 minutes, the imprinted thin layer was torn off with tweezers and observed under an Olympus BX61 universal microscope (Olympus, Japan) directly after pressing with a cover slip. The stomata numbers were counted by observing four visual fields per leaf. The mean value of the stomatal density (per mm2) was calculated. The guard cell length was measured for 20 stomata per leaf, and the mean stomatal length was calculated.
Observation of the Tissue Structure of the Leaf Cross Section
Paraffin sections were prepared according to the method of Sun et al. (2010). Mature spring shoot leaves were cut into 0.3 × 1-cm pieces with a blade fixed in FAA (ethanol:acetic acid:formaldehyde = 90:5:5), dehydrated in a gradient ethanol series (30 ‑ 100%), and embedded in paraffin. Then, the embedded materials were sliced with a Leica Ultracut R ultrathin slicing machine (Leica, Bensheim, Germany) to 10-µm thickness. Stained paraffin sections were observed with an Olympus BX61 universal microscope (Olympus, Japan). The upper epidermis, lower epidermis, palisade tissue, and total leaf thickness were measured in the micrographs.
Scanning Electron Microscopy
According to the method of Han et al. (2018), fresh pollen was pretreated on the sample stage and sprayed with the 108 Auto Cressington Sputter Coater (Cressington, UK). The samples were examined with a scanning electron microscope (JSM-6390/LV, NTC, Japan), and representative images were obtained.
Flow Cytometric Analysis
The ploidy level of HH was determined by flow cytometry according to the method of Zhang et al. (2007). Approximately 2 cm2 of leaf was chopped with a razor blade in a Petri dish in 1.5 mL of HR-A nuclei extraction solution (Partec High-Resolution Staining Kit; Partec GmbH, Münster, Germany). After adding 2 mL of HR-B DAPI staining solution (Partec High-Resolution Staining Kit), the mixture was filtered through a 30-µm nylon filter. Fluorescence of the nuclei was measured with a CA-II flow cytometer (Partec, Germany). Young leaves of CH and OW were used as the controls.
High-Performance Liquid Chromatography Carotenoid Analysis
The carotenoid extraction and saponification processes were performed under the condition of weak or dark light to avoid decomposition. The carotenoid pigments were analyzed by reversed phase high-performance liquid chromatography (HPLC) using the modified binary gradient elution procedure originally developed by Lee et al. (2001). The forms of the carotenoids were identified by the retention time of the standard sample and the characteristic spectral value. Standard samples (β-cryptoxanthin, violaxanthin, and lutein) were purchased from CaroteNature (Lupsingen, Switzerland).
DNA Extraction and Simple Sequence Repeat Analysis
DNA extraction and SSR analysis were conducted based on Cheng et al. (2003a, 2005). PCR products were mixed with an equal volume of formamide loading buffer (98% formamide, 10 mM EDTA pH 8.0, 0.025% Bromophenol Blue, and xylene cyanol). Two milliliters of mixture of the equipartition sample was added to 12% polyacrylamide gel and electrophoresed for 5 hours at 180 V. Bands were displayed after silver staining and recorded on a ScanMaker 3830 (Microtek, Shanghai, China). Primers used for nuclear simple sequence repeat (nSSR) analyses were as described by Xu et al. (2013). Fourteen chloroplast simple sequence repeat (cpSSR) primers (SPCC1 to SPCC14) were utilized to analyze chloroplast genomes according to the report of Cheng et al. (2003b). One mitochondrial simple sequence repeat (mtSSR) primer was used to analyze mitochondria genomes according to the report of Cheng et al. (2003c).
Statistical Analysis
Data are reported as means ± standard error (SE). Statistical analyses were performed with SAS Statistics 9.1 (SAS Institute Inc, NC, USA). Means followed by different letters are significantly different at p = 0.01 according to least significant difference test.
Results
Morphological Characteristics of the Fruit
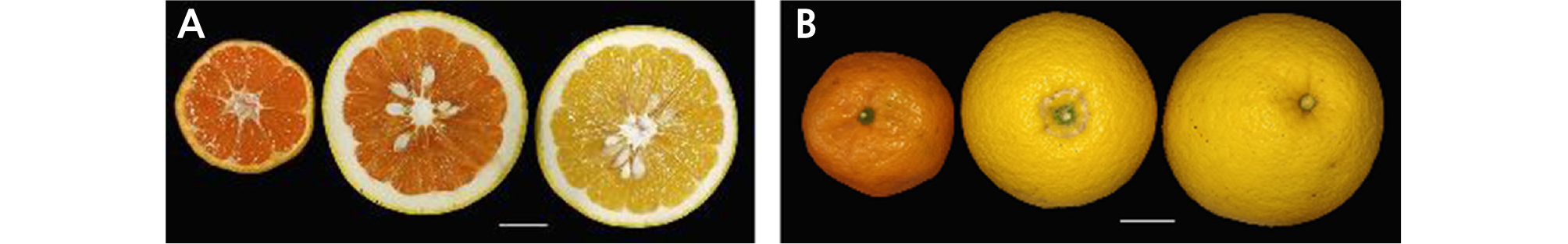
The fruit index and shape of HH were similar to those of OW and were markedly different from those of CH (Table 1 and Fig. 1A). However, the fruit width (9.67 cm) and height (8.05 cm) both exceeded those of its donors. The fruit of HH (384.88 g) was heavier, but the edible rate (67.05%) was less than that of both donors. The flesh weight (257.12 g) of HH was similar to that of CH. During the experiment, we found that the pericarp of HH was thicker than that of its donor plants, which might lead to considerable storage quality. The color of the juice sacs in HH (deep orange) was similar to that of OW (Table 1 and Fig. 1B), and the peel had a strong aroma similar to that of CH. Additionally, HH was seedy with an average of 19.82 seeds per fruit, which was similar to that of CH.
Table 1. Morphological characteristics of fruit between 'Hongrou Huyou' and its donor plants
Leaf Morphology
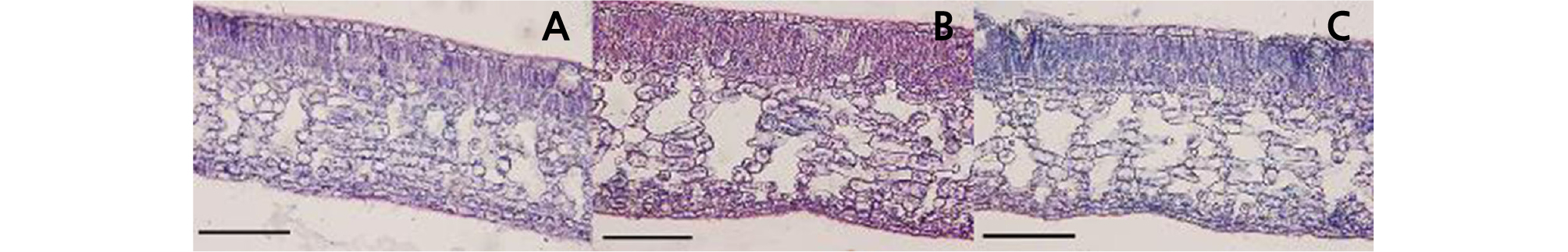
The leaf shape of HH was similar to that of CH, and their petiole wings were obviously larger than those of OW (Table 2 and Fig. 2). The leaf length of HH (10.44 cm) was shorter than that of its donors. The leaf width of HH (5.26 cm) was broader than that of OW (4.67 cm) but narrower than that of CH (5.86 cm). The stoma density and length of HH were similar to those of OW and varied compared to those of CH (Table 2 and Fig. 3). According to the observation of leaf blade paraffin sections, the palisade tissue thickness of HH was 122.91 µm, which was significantly larger than that of OW (80.20 µm) and CH (97.91 µm). The thickness of the upper epidermis of HH was 25.00 µm, which was thicker than that of OW (19.79 µm) and CH (19.80 µm). However, the thickness of the lower epidermis was 37.50 µm, which was similar to that of OW (36.46 µm) but significantly different from that of CH (32.29 µm). The total leaf thickness of HH was 539.58 µm, which was significantly larger than that of OW and CH (Table 2 and Fig. 4).
Table 2. Morphological characteristics of leaf between 'Hongrou Huyou' and its donor plants
Morphology of Mature Pollen using Scanning Electron Microscopy
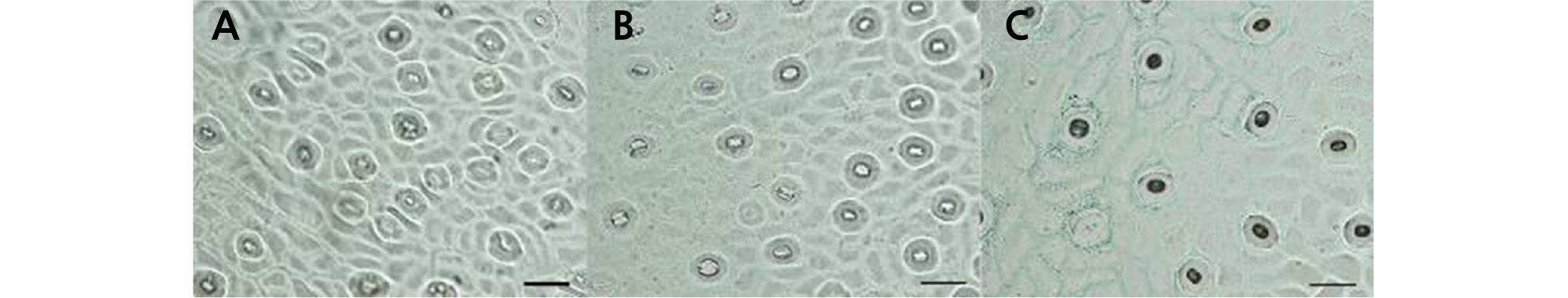
Through scanning electron microscopy (SEM), we observed that the size, morphology, and ornamentation of the pollen exine of HH were similar to those of CH but different from those of OW (Fig. 5). The SEM analysis showed that the pollen of HH and CH was cuboid, with an equatorial axis of 37 ‑ 39 µm and polar axis of 24 ‑ 26 µm. The polar view was four lobed squares with four long and deep colporates. The pollen exine surface was rough, with many round cavernous ornaments (Fig. 5C ‑ 5F). The pollen of OW was nearly globular, with an equatorial axis of 26 ‑ 28 µm and polar axis of 24 ‑ 26 µm and was four-lobed globose in the polar view (five-lobed globose in a few cases) with four or five short colporates. The pollen exine surface was smooth, with a small number of circular cavernous ornaments of uneven size (Fig. 5A and 5B).
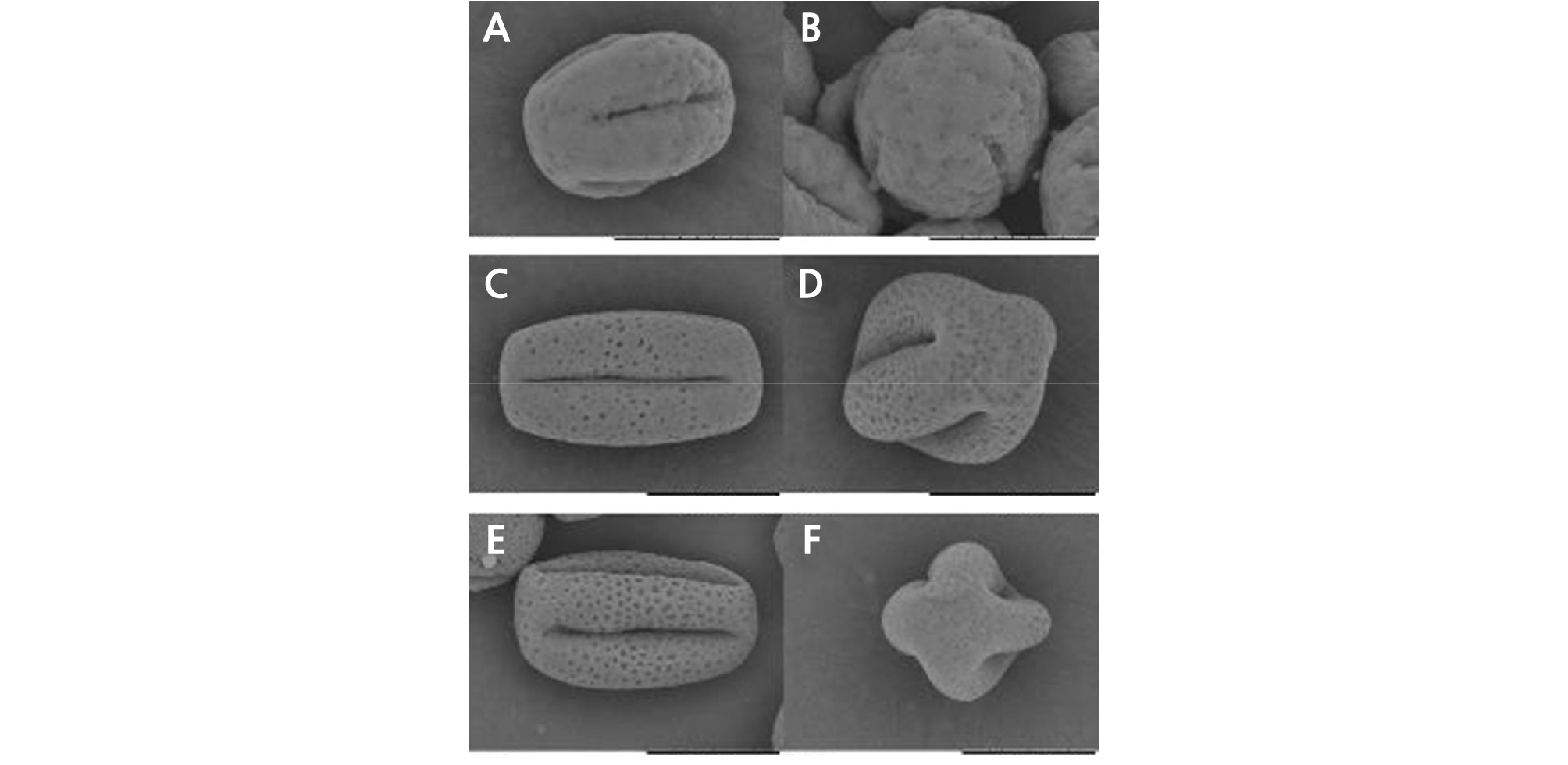
Fig. 5.
Pollen morphology of ‘Hongrou Huyou’ and its two donors (bars = 20 µm). (A) Pollen of ‘Owari’ satsuma mandarin, equatorial view, (B) pollen of ‘Owari’ satsuma mandarin, polar view, (C) pollen of ‘Hongrou Huyou’, equatorial view, (D) pollen of ‘Hongrou Huyou’, polar view, (E) pollen of ‘Changshan-huyou’, equatorial view, (F) pollen of ‘Changshan-huyou’, polar view.
Ploidy Determination
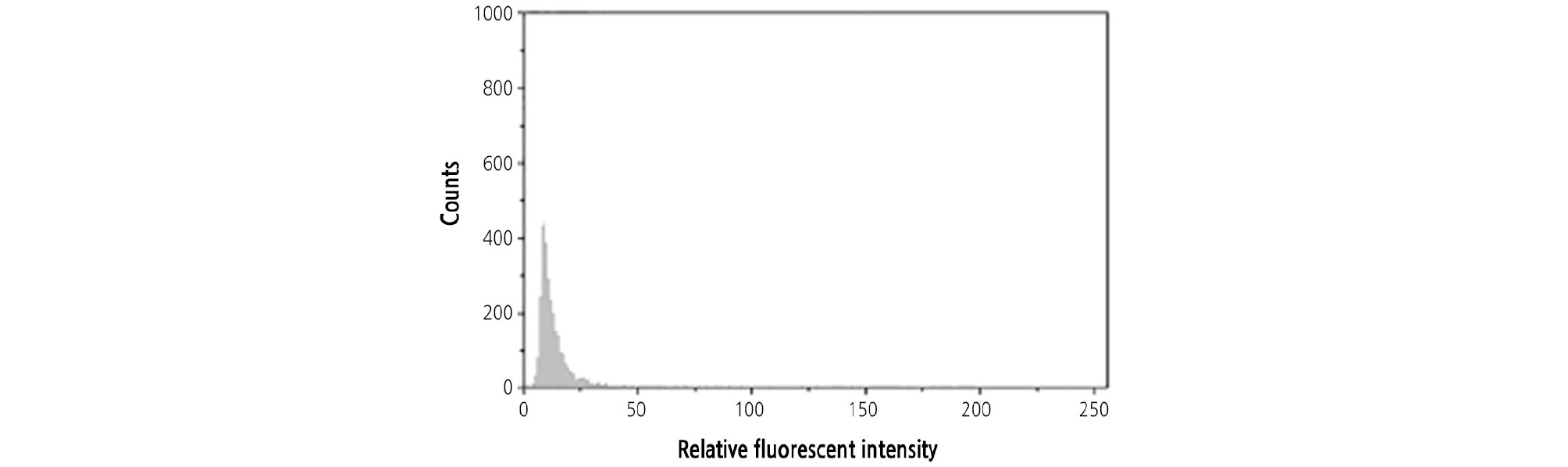
Both CH and OW were diploid. The ploidy of HH was detected by flow cytometry with CH and OW as the controls. When the leaves of HH and its two donors were mixed and chopped together, only one peak was found by flow cytometry, indicating that the ploidy of HH was naturally in diploid (Fig. 6).
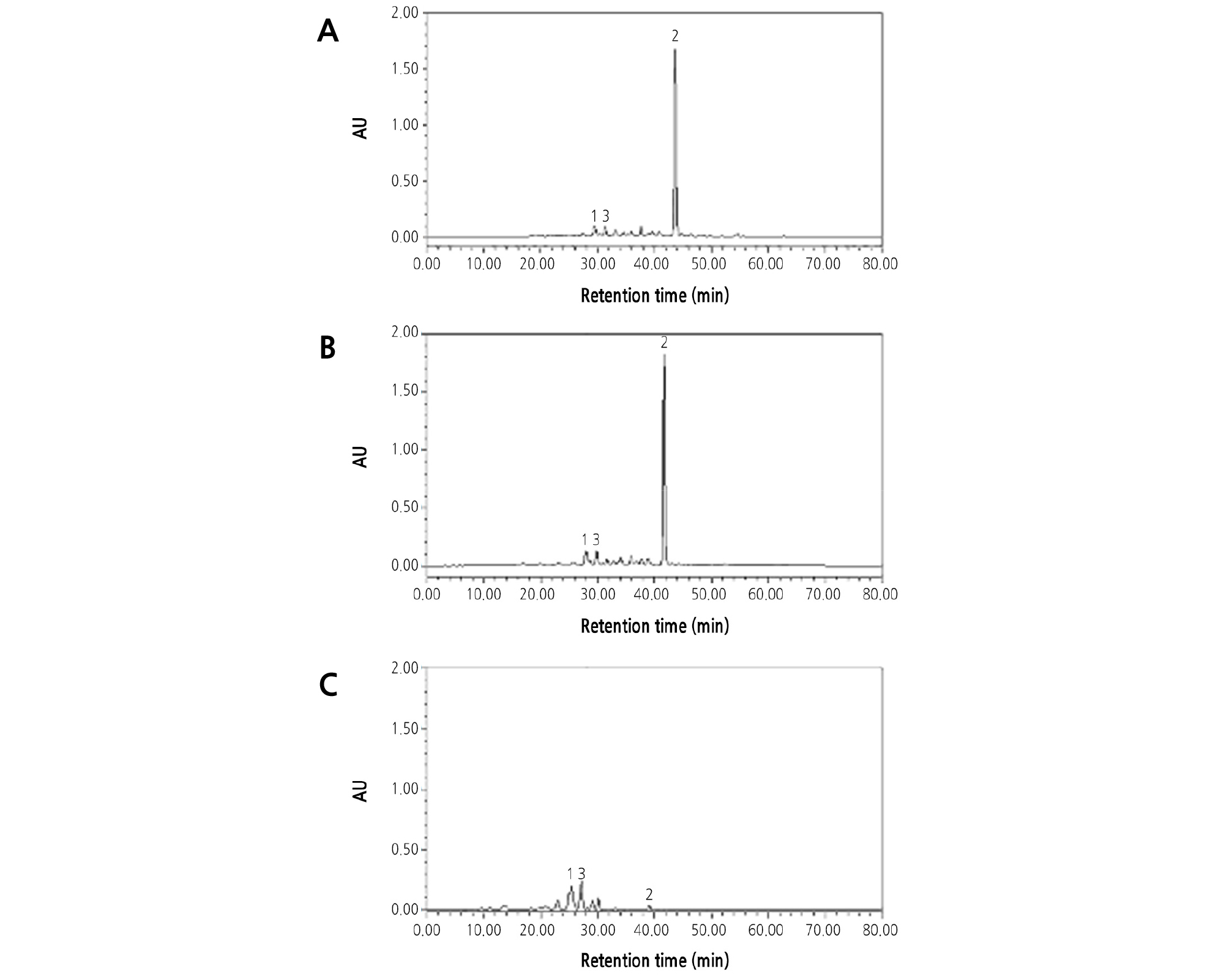
β-Cryptoxanthin was the main carotene in the flesh of OW (Fig. 7A), which caused the deep-orange flesh color. Lutein and violaxanthin were the two main carotenoids in CH (Fig. 7C), which caused the yellow flesh color. The HPLC analysis showed that both the carotene composition and content in the flesh of HH were very similar to those of OW and that β-cryptoxanthin was the predominant carotene accumulated in the juice sac (Fig. 7A and 7B).
Characterization of the Nuclear, Chloroplast, and Mitochondrial Genomes
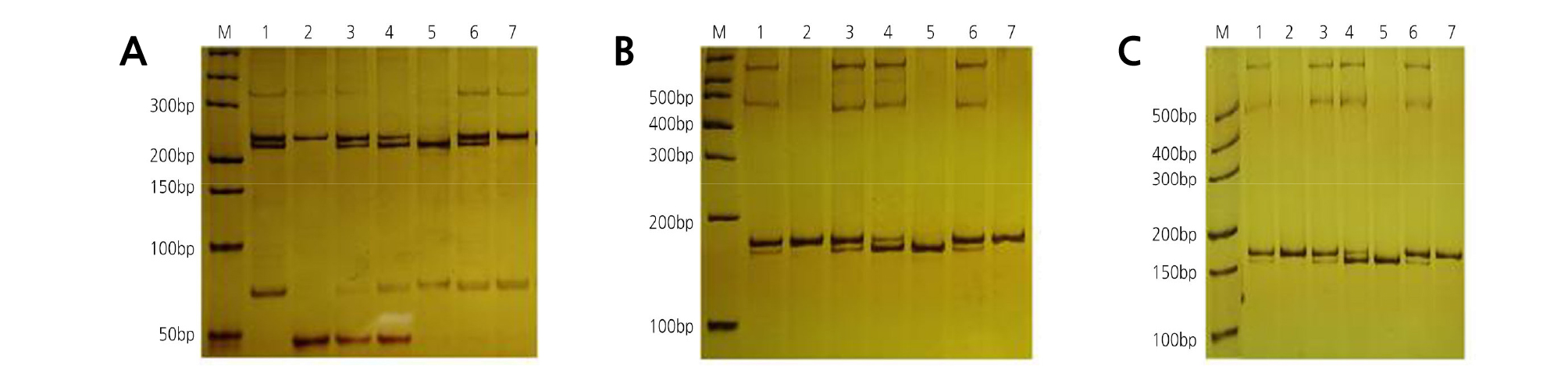
The nuclear genomes of the chimera and its donors were analyzed using 45 nSSR primer pairs. OWandCH had a distinguishable band in their nuclear genomes when amplified with primer Csin.0078 (Table 3). To determine the chloroplast genomes of the chimera and its donors, 14 pairs of cpSSR primers (SPCC1 to SPCC14) were used. Among them, SPCC9 (Table 3) revealed polymorphism and specificity between OWandCH. Meanwhile, primer M7 (Table 3) revealed polymorphism and specificity between OWandCH in the mitochondrial genomes. In the nuclear (Fig. 8A), chloroplast (Fig. 8B), and mitochondrial (Fig. 8C) genomes, the amplified products of HH shared specific bands with both donors, which demonstrated that the grafted chimera contained the nucleus, chloroplast, and mitochondrial genomes from both donors. In addition, the outer pericarp (epidermis and flavedo), segment wall, and juice sac of HH contained the bands of OW and CH, whereas the albedo contained only the bands of CH.
Table 3. Selected primers for SSR
Discussion
The serial analysis in this study confirmed that HH was a natural grafted chimera with stomatal morphology, stomatal density, and pulp characteristics similar to OW, suggesting that OW (the stock) contributed to the L1 of HH. The other characteristics, including the petiole wing, fruit size, aroma, and color of the rind, seed, and pollen, were much similar to CH, indicating that L2 and L3 originated from CH (the scion). In previous studies, some citrus-grafted chimera varieties were discovered, such as ‘Zaohong’ navel orange (‘Robertson’ navel orange and satsuma mandarin) (Zhang et al., 2007), ‘Hongrou Taoye’ (‘Taoye’ sweet orange and satsuma mandarin) (Zhang et al., 2015), and ‘NFF’ and ‘FNN’ (‘Fukuhara’ sweet orange (F) and ‘Kawano’ natsudaidai (N)) (Ohtsu and Kuhara, 1994). Therefore, HH is a new chimera composed of CH and OW.
In this study, total DNA was extracted from whole intact leaves, since the epidermis of the leaf came from L1 and the mesophyll came from L2 and L3 (Frost and Krug, 1942; Tilney-Bassett, 1986). However, the fruits of HH were divided into four parts to enable accurate positioning of the origin of each tissue. Thus, our nSSR, cpSSR, and mtSSR analyses indicate that the leaves of HH possess nuclear, chloroplast, and mitochondrial genomes from both donors. Because the chloroplast and mitochondrial genomes are a maternal inheritance, citrus hybrid offspring only possesses these two genomes from their female parent. However, in somatic hybrids, the chloroplast and mitochondrial genomes originate from only one donor plant (Cheng et al., 2003b, 2003c). In this study, the chloroplast and mitochondrial genomes of HH were derived from both donors. Therefore, we confirmed that HH was a chimera. Additionally, relevant studies have shown that the contribution rates of donor plant cells (L1, L2, and L3) in different tissues and organs are different (Stewart and Dermen, 1975). Similarly, in this study, the DNA bands from CH were less intense than those from OW in the outer pericarp, segment wall, and leaf of HH, whereas the DNA bands were more intense in the juice sac of HH, which also illustrated this phenomenon.
HH possessed the rich aroma (similar to CH) and ease of peeling (similar to OW) traits of its donors. The flesh traits of HH were similar to those of OW, but its taste mixed with the aroma of CH. During citrus fruit development, the skin of the juice sacs is produced by the endocarp, and the hypodermis of the endepidermis continues to divide and form the club-shaped juice sac (Sugiyama and Sakaguchi, 2002). Ohtsu and Kuhara (1994) demonstrated that the juice sac of citrus chimeras developed from the L1 cell layer. Similarly, Zhou et al. (2002) suggested that the juice sacs of the citrus chimeras ‘NFF’ and ‘FNN’ were L1 derivatives. According to previous studies (Ohtsu and Kuhara, 1994; Zhou et al., 2002), the juice sacs of HH should only possess genetic material from OW; however, we observed that HH combined the DNA bands from both of its donors in the nSSR, cpSSR, and mtSSR molecular analyses. Juice cells may develop from codevelopment of L1 and L2/L3 or from L1, L2, and L3, which is different from the classical conclusion that L1 layer cells develop into juice cells. In future research, we need to determine which cell layers develop into juice cells, and we can purposefully select grafting donors to synthesize grafting chimera.
HH expresses the qualified characteristics from its donor plants and presents intermediate characteristics. Its flesh, deep-orange in color, juicy, soft, tender, and rich in aroma, is often used in fresh food and juicing. It can be stored at room temperature until April to May of the following year. HH was reproduced by bud graft breeding, and its agronomic characteristics were stable and maintained for decades. Therefore, we suggest that the new grafted citrus chimera HH can serve as a distinctive variety for the citrus fresh market.