Introduction
Materials and Methods
Plant Material
Histological Analysis of Pollen Development
Quantifying Pollen Production
Pollen Viability and Germination Rate
Statistical Analysis
Results
Pollen Development in Staminate Flowers
Quantifying Pollen Production
Pollen Viability and Germination Rate
Discussion
Introduction
Kiwifruit is a perennial, deciduous fruit vine that belongs to the genus Actinidia. It consists of approximately 54 species and 21 cultivated varieties, a total of 75 taxa including the commercially important A. chinensis var. deliciosa and A. chinensis. var. chinensis (Huang et al., 2004; Huang, 2014; Oh et al., 2019). Kiwifruit is a dioecious plant, with distinct male and female individuals. Male plants have staminate flowers, whereas female plants have pistillate flowers. There are well-known distinctive morphological and functional differences between staminate and pistillate flowers (Schmid, 1978; Ferguson, 1984). In general, staminate flowers are small and develop normal anthers with fertile pollen grains and an abnormal ovary, which lacks ovules, a style, and a stigma. However, pistillate flowers are large and develop a normal ovary with fertile ovules and abnormal anthers with non-viable pollen grains.
Kiwifruits are mainly grown in the area of Jeju and in the southern coastal regions of Korea, and they are regarded as the second-largest fruit crop after citrus in Jeju (JSSGP, 2018). They are grown mostly in plastic film houses in Jeju, whereas in other regions they are grown in open fields. Major kiwifruit cultivars consist of the yellow-fleshed kiwifruit ‘Sweet Gold’ and ‘G3’, the green-fleshed kiwifruit ‘Hayward’, and the red-fleshed kiwifruit ‘Hongyang’ in Jeju, which are all pistillate cultivars. These pistillate kiwifruit cultivars require artificial pollination for commercial production. In addition, high pollen viability and germination and being free of pathogen contamination are essential for ensuring high yield and good fruit quality (Seal et al., 2013b). Most commercial pollen used in Korea is imported from China because of its low cost and availability. Although New Zealand started to domesticate and commercialize kiwifruits in the 20th century, China is the center of Actinidia origin and has the greatest diversity of wild germplasm and other genetic resources, such as diverse polyploids (Huang, 2014). Kiwifruits have varying ploidy levels, ranging from diploid to octoploid (Watanabe et al., 1990; Yan et al., 1997; Seal et al., 2013a; Huang, 2014). In general, the green-fleshed kiwifruit, A. chinensis var. deliciosa ‘Hayward’ is known to be hexaploid, whereas the yellow-fleshed kiwifruit, A. chinensis var. chinensis ‘Sweet Gold’ and red-fleshed kiwifruit ‘Hongyang’ are known to be tetraploid and diploid, respectively (Warrington and Weston, 1990; Li et al., 2010; Huang, 2014; Jeong et al., 2018).
In recent years, it has increasingly been reported that pollinizers with varying ploidy levels have different effects on fruit yield and characteristics (Li et al., 2010; Jeong et al., 2018; Stasiak et al., 2019). In addition, kiwifruit bacterial canker (Pseudomonas syringae pv. actinidiae), the most serious and dangerous disease in kiwifruit, may be dispersed via pollen during artificial pollination (Donati et al., 2018). Despite the inadequate information regarding the specific ploidy and whether pollen should be tested for bacterial canker, pollen from China continues to be an important genetic resource and a source of pollination in Korea. Recently, interest in self-production of kiwifruit pollen has greatly increased in Korea. However, to date, there have been few studies on kiwifruit pollen development and timing of pollen collection in Korean kiwifruit production regions. Therefore, this study was conducted to determine the optimum timing for pollen collection through investigating the pollen development at progressive flower developmental stages of the two staminate cultivars ‘Bohwa’ and ‘Chieftain’, which are the major pollinizers used in the province of Jeju, Korea.
Materials and Methods
Plant Material
Two staminate cultivars of A. chinensis var. deliciosa ‘Bohwa’ and ‘Chieftain’, were used in a 2-year study from 2017 to 2018. ‘Bohwa’ was bred in Korea, whereas ‘Chieftain’ was bred in New Zealand, both being hexaploid cultivars. Vines were grown in an unheated, plastic film house, which was located in Jeju, Korea. All cultural practices, such as pruning, fertilization, and pest control, were performed under conventional management.
Histological Analysis of Pollen Development
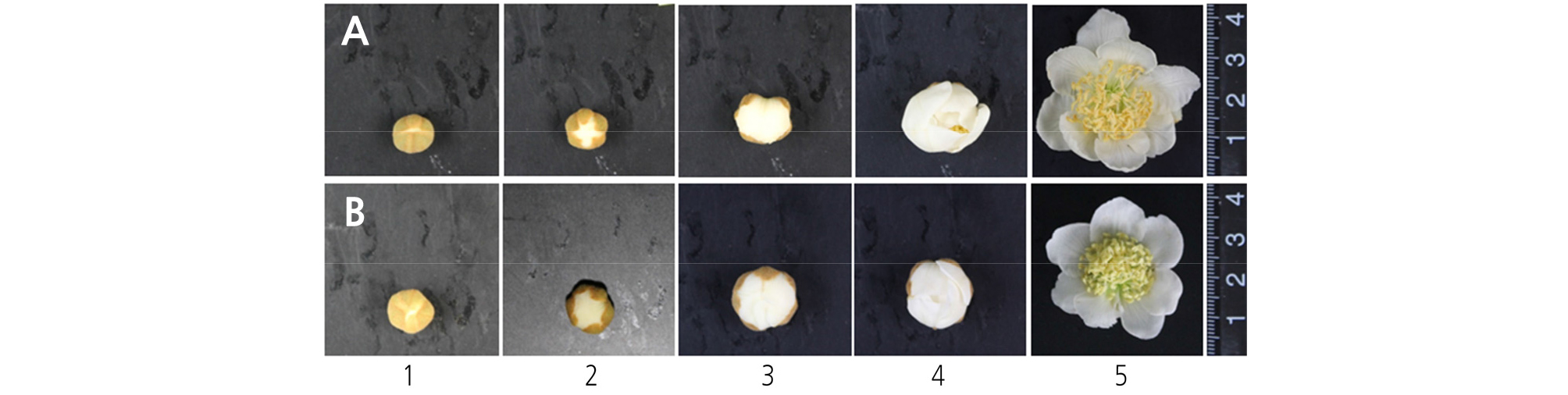
Staminate flowers were classified into five progressive developmental stages according to flower morphology, with the first stage occurring when white petals first became visible and the fifth stage when the petals were completely open and in full bloom (Figs. 1 and 2). The anthers were collected at each developmental stage and immediately fixed using a fixative solution of 0.05 M phosphate buffer mixed with 2.5% glutaraldehyde and 6% paraformaldehyde at 4°C, followed by incubation for 48 h in the dark. The fixed samples were then dehydrated in an ethanol series. During the dehydration process, the air in the pericarp tissue was removed four times using a vacuum pump (VS-975M; Vision Co., Korea), and the samples were stored at 4°C for 24 h in the dark. The samples were then embedded with a Technovit 7100 (Heraeus Kulzer, Germany) following the method described by Yeung (1999). The embedded blocks were then cut to a thickness of 7 mm using a microtome (RM2165; Leica, Germany), and the transverse sections were mounted on glass slides and examined under a fluorescence microscope (Leitz DMRBE; Leica) after staining with 4', 6-diamidino-2- phenylindole (DAPI).
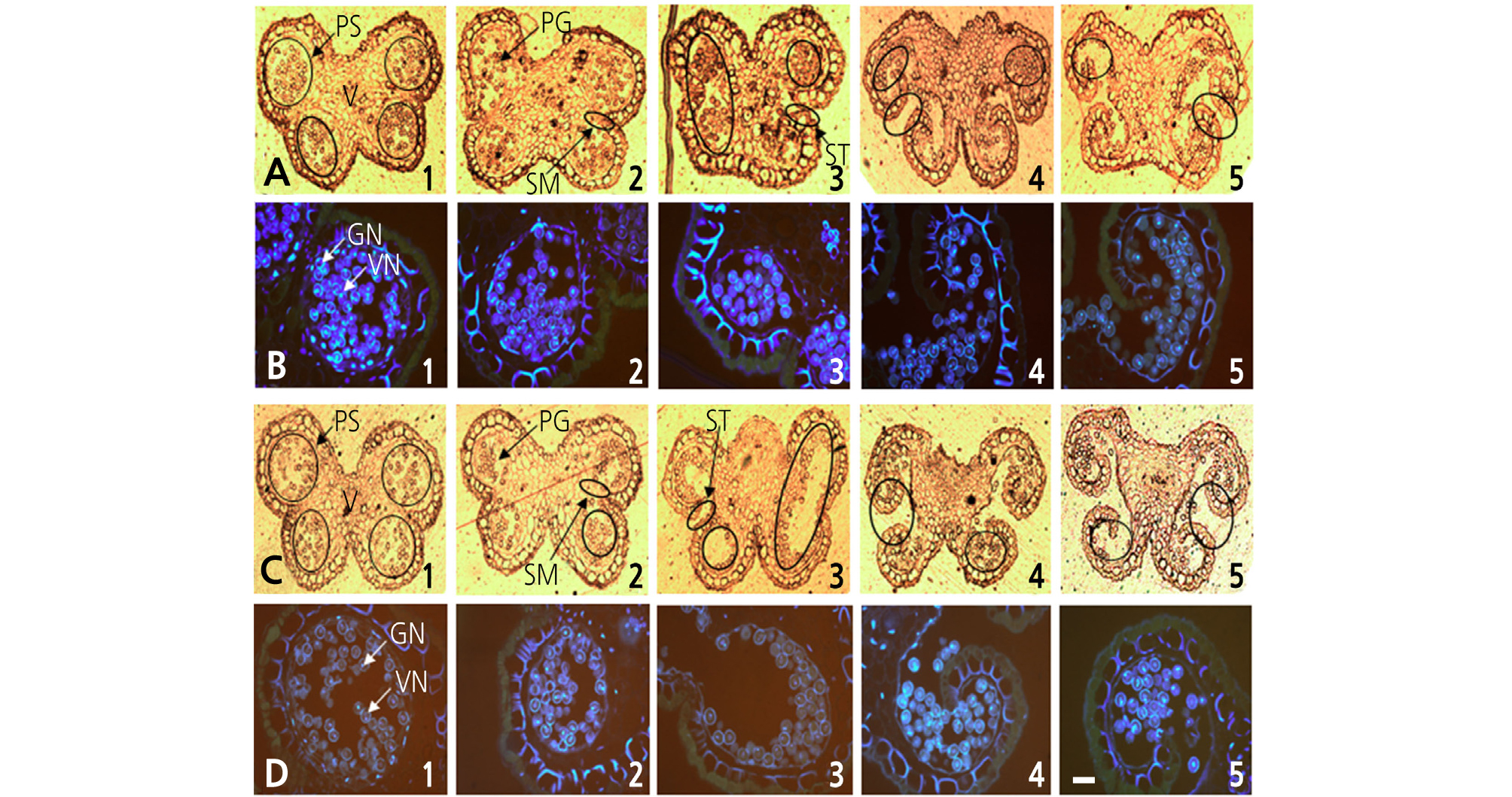
Fig. 2.
Characterization of pollen development in kiwifruit staminate flowers of ‘Bohwa’ (A1–A5, B1–B5) and ‘Chieftain’ (C1–C5, D1–D5) at different stages of flower development. (A1–A5, C1–C5), observed under normal light; (B1–B5, D1–D5), stained by DAPI and observed under fluorescent light; (B1-B2, D1-D2), midbicellular pollen; (B3, D3), late bicellular pollen; (B4-B5, D4-D5), mature pollen at full dehiscence. PS, pollen sac; V, vascular bundle; PG, pollen grain; SM, septum; ST, stomium; VN, vegetative nucleus; GN, generative nucleus. The scale bar indicates 100 µm.
Quantifying Pollen Production
One kilogram of staminate flowers at each flower developmental stage was collected. The anthers, petals, calyx, and peduncle of each flower were separated and removed using separation equipment (SWX-400; Samwoo Engineering, Korea). The separated anthers were then dried in a forced-draft static drier (SWX-6000; Samwoo Engineering) at 25°C for 24 h, and pollen grains were separated and collected from the dried anthers using a pollen collector (SWX-6000; Samwoo Engineering). The amount of pollen produced at each flower developmental stage was measured on the basis of weight.
Pollen Viability and Germination Rate
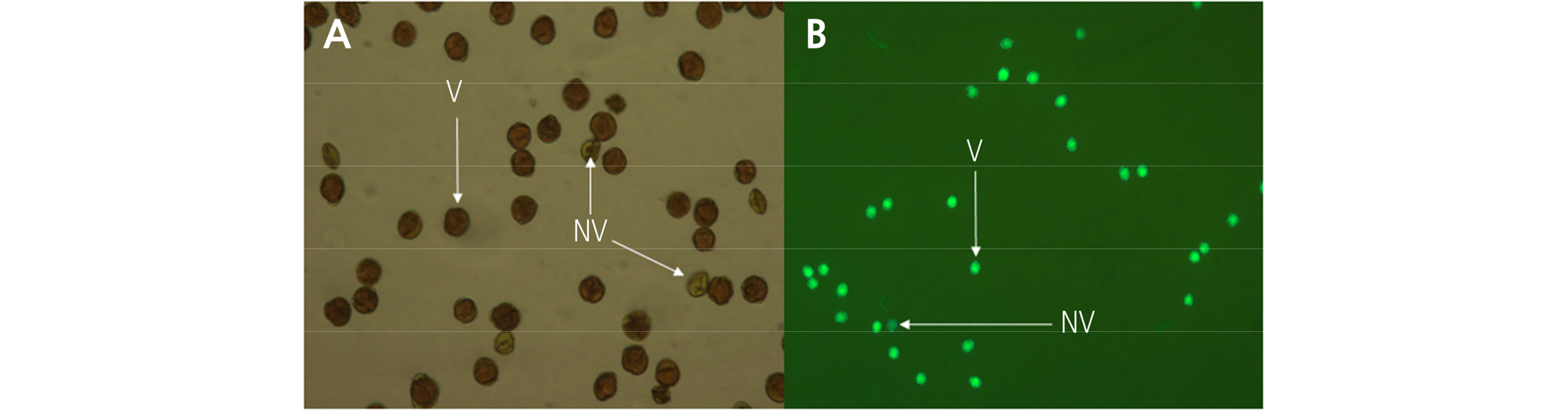
The pollen viability at the 2nd to 5th developmental stages was assessed using fluorescein diacetate (FDA) and 1% iodine potassium iodide (I2KI) staining methods as described by Pok et al. (2015). Using light and fluorescence microscopy systems (Leitz DMRBE; Leica), for the I2KI staining, dark-stained pollen grains were considered to be viable; and for the FDA staining, green-fluorescing pollen grains were considered to be viable (Fig. 3). To assess pollen germination, pollen grains were collected from each flower at the 2nd to 5th developmental stages, suspended in liquid germination media consisting of 10% sucrose and 0.01% H3BO4, and allowed to germinate at 22°C for 3 h in dark. Pollen grains were considered germinated when the pollen tube length was two times longer than the size of pollen grain.
Statistical Analysis
The data was analyzed using IBM SPSS Statistics (Version 18; IBM Corp., Armonk, NY, USA). To evaluate the significance of differences between mean values, Duncan’s multiple range test was applied at the significance level of p < 0.05.
Results
Pollen Development in Staminate Flowers
Pollen development was analyzed across five progressive developmental stages, from the 1st stage when white petals first became visible with the calyx to the fifth stage when all petals were completely unfolded and in full bloom. Two staminate cultivars, ‘Bohwa’ and ‘Chieftain’, showed a similar pattern in terms of pollen development, with the exception being that the pollen development in ‘Chieftain’ progressed slightly quicker (Fig. 2). From the first to second flower developmental stage, tapetum tissues began to degenerate and pollen grains developed into a binuclear stage (Fig. 2A1, 2A2, 2B1, 2B2, 2C1, and 2D1). The septum was degraded at the 2nd to 3rd developmental stages (Fig. 2A3, 2B3, 2C2, 2C3, 2D2, and 2D3). At the 4th developmental stage (full balloon stage, just before petal opening), the flowers started disclosing anthers with a partially opened stomium and pollen grains started to dehisce from the disclosed part (Fig. 2A4, 2B4, 2C4, and 2D4). At the 5th developmental stage (in full bloom), the stomia of most anthers were opened completely and dry pollen grains began to dehisce (Fig. 2A5, 2B5, 2C5, and 2D5).
Quantifying Pollen Production
The potential of pollen production was assessed for two consecutive years (Table 1). The largest quantity of pollen was obtained at the full balloon stage corresponding to the 3rd and 4th stages of flower development in ‘Bohwa’ and ‘Chieftain’, respectively. ‘Bohwa’ showed increased pollen production ranging between 1.4 and 1.8 times at the 3rd stage of flower development (early balloon stage) to the 5th stage of flower development (in full bloom) compared to ‘Chieftain’
Table 1. The amount of pollen production at different developmental stages of flowers of two staminate kiwifruit cultivars 'Bohwa' and 'Chieftain'
zMean separation within columns performed by Duncan's multiple range test at the 5% level.
Pollen Viability and Germination Rate
The pollen viability of ‘Bohwa’ and ‘Chieftain’ was assessed from the 2nd to the 5th stage of flower development (Table 2). I2KI staining showed higher pollen viability compared to FDA staining in both staminate cultivars. ‘Bohwa’ appeared to have slightly higher pollen viability with ranges of 91.8–94.5% and 83.3–91.1% compared to ‘Chieftain’ with ranges of 90.1–93.2% and 75.9–85.9% using I2KI staining and FDA staining, respectively. Moreover, the only significant difference in pollen viability among the flower developmental stages resulted from the FDA staining in ‘Bohwa’.
Table 2. The percentage of pollen viability at different developmental stages of flowers of two staminate kiwifruit cultivars 'Bohwa' and 'Chieftain'
zMean separation within columns performed by Duncan's multiple range test at the 5% level.
Pollen germination was assessed by incubating pollen grains suspended in liquid germination medium. The germination rate ranged from 7.1 to 65.0% and from 10.2 to 68.7% in ‘Bohwa’ and ‘Chieftain’, respectively, depending largely on the flower developmental stage (Table 3). The highest pollen germination rate was obtained in full bloom (the 5th stage of flower development). However, there was no statistically significant difference in pollen germination between the 4th and 5th stages of flower development.
Table 3. The percentage of pollen germination at different developmental stages of flowers of two staminate kiwifruit cultivars 'Bohwa' and 'Chieftain'
zMean separation within columns performed by Duncan's multiple range test at the 5% level.
Discussion
This study was conducted to determine the optimal timing of pollen collection by investigating pollen developmental characteristics and the amount of pollen production in two staminate kiwifruit cultivars, ‘Bohwa’ and ‘Chieftain’, the most common pollinizer cultivars in Jeju, Korea. The pollen development at each flower developmental stage was similar in both ‘Bohwa’ and ‘Chieftain’ (Figs. 1 and 2). However, there was a slight difference in the timing of stomia separation between ‘Bohwa’ (Fig. 2A4) and ‘Chieftain’ (Fig. 2C3), which indicates that anther dehiscence and pollen shedding may occur earlier in ‘Chieftain’ compared to ‘Bohwa’. The data support this since pollen collection decreased by 23% and 38% at the 5th stage of flower development in ‘Bohwa’ and ‘Chieftain’, respectively (Table 1). At the first developmental stage, microspores at the end of mitosis and their development into early pollen with two nuclei (vegetative nucleus and generative nucleus) were identified (Fig. 2A1, 2B1, 2C1, and 2D1). At the 2nd to 3rd developmental stages, the septa were shown to degenerate and pollen sacs merged (Fig. 2A2, 2A3, 2D2, and 2D3). At the 4th developmental stage, the full balloon stage, the stomia dehisced and mature pollen grains were ready to be shed prior to the petals opening. Hopping (1990) indicated that pollen shedding commences as petals begin to unfold and that more than half of the pollen is lost by the time the petals fully unfold, but not much pollen loss was seen in this study. This may be due to the use of different cultivars. Pok et al. (2015) also reported that some Korean landrace citruses exhibited a mitotic pollen stage just at the full balloon stage, which was not in agreement with the results of this study. This study showed that kiwifruit pollen may mature earlier compared to other fruit crops, such as citrus, and pollen shedding may occur in advance of the petals opening depending on the staminate cultivars.
The pollen production of ‘Bohwa’ and ‘Chieftain’ was assessed for two consecutive years at different flower developmental stages. The highest amount of pollen production was obtained from staminate flowers at the 4th developmental stage (full balloon stage) of both ‘Bohwa’ and ‘Chieftain’ (Table 1). Thereafter, the pollen production decreased by 23% and 38%, respectively, in both staminate cultivars. Histological analysis showed that stomium dehiscence begins at the 3rd or 4th stage of flower development in ‘Bohwa’, but at the 3rd developmental stage in ‘Chieftain’ (Fig. 2). Stomium dehiscence is a decisive indicator of anther opening that is distinctly associated with pollen shedding and a decrease in pollen production. The quantity of pollen collected from ‘Bohwa’ was about two times greater than that from ‘Chieftain’ at the three different stages of flower development, from the 3rd stage (early balloon stage) to the 5th stage (fully open flower), which is related to a higher number of stamens, about twice as many, in ‘Bohwa’ (Kwak et al., 2009).
Evaluating pollen viability is important not only in assessing seed formation, but also for crop improvement, breeding programs, and other aspects of pollination science (Stanley and Linskens, 1974). Pollen viability can be assessed using different staining methods (Devi et al., 2015; Pok et al., 2015). In this study, two staining methods (i.e., FDA staining and I2KI staining) were applied to assess pollen viability in staminate cultivars. I2KI staining showed slightly higher pollen viability compared to FDA staining (Table 2), which was similar to what was reported by Pok et al. (2015). At all flower developmental stages, higher pollen viability was found in ‘Bohwa’, which ranged from 91.8 to 94.5% after I2KI staining and from 83.3 to 91.0% after FDA staining, compared to “Chieftain,” which ranged from 90.1 to 93.2% after I2KI staining and from 75.9 to 85.9% after FDA staining. These pollen viabilities were similar to the results reported by Devi et al. (2015) in ‘Allison’ and ‘Tomuri’ cultivars, in which 93–95% pollen viability was measured following 1% acetocarmine staining or 1% tetrazolium chloride staining, whereas about 85% pollen viability was measured following 0.1% erythrosine B staining. Pollen germination increased from 7.1–10.2% at the 1st stage of flower development (at which point white petals start being visible) to 65.0–68.7% at the 5th developmental stage (fully open flower) (Table 3). Devi et al. (2015) reported that >50% pollen germination is enough for normal fruit set and development in four pistillate cultivars, including ‘Hayward’. Therefore, the results of this study indicate that the 4th and 5th stages of flower development (full balloon stage and fully open flower, respectively) are more than adequate for conventional pollination and commercial fruit production. Pollen germination has generally been reported to range from 65 to 75% in mid- to late-season cultivars; however, a slightly higher rate was described in early-season cultivars from shake culture test (Hopping, 1990). The pollen germination rates reported in the literature for mid- to late-season cultivars are in accordance with the results of this study, especially since ‘Bohwa’ is a late-season cultivar.
In conclusion, adequate pollen production and germination rates are some of the most important factors in the commercial production of kiwifruits because of their association with sufficient fruit set and good fruit quality. In this study, pollen development, production quantity, viability, and germination were evaluated with the aim of determining the optimum timing of flower and pollen collection from two staminate cultivars, ‘Bohwa’ and ‘Chieftain’, which are commonly used as pollinizers in Jeju, Korea. Considering all of these parameters, the results indicate that the optimum timing of pollen collection is at the 4th stage of flower development (full balloon stage). Moreover, the 3rd (early balloon stage) and 5th (fully open flower) developmental stages of staminate flowers in both ‘Bohwa’ and ‘Chieftain’ cultivars also can be used in pollen preparation. Despite the limited number of cultivars evaluated, the results also suggest that similar timing of pollen preparation could be applicable for other staminate cultivars.